Best Of The Best Tips About How To Increase Concentration Of A Solution
For example, if the percent solution under consideration is to be used at widely different temperatures, then it is better to prepare the solution as a weight/weight.
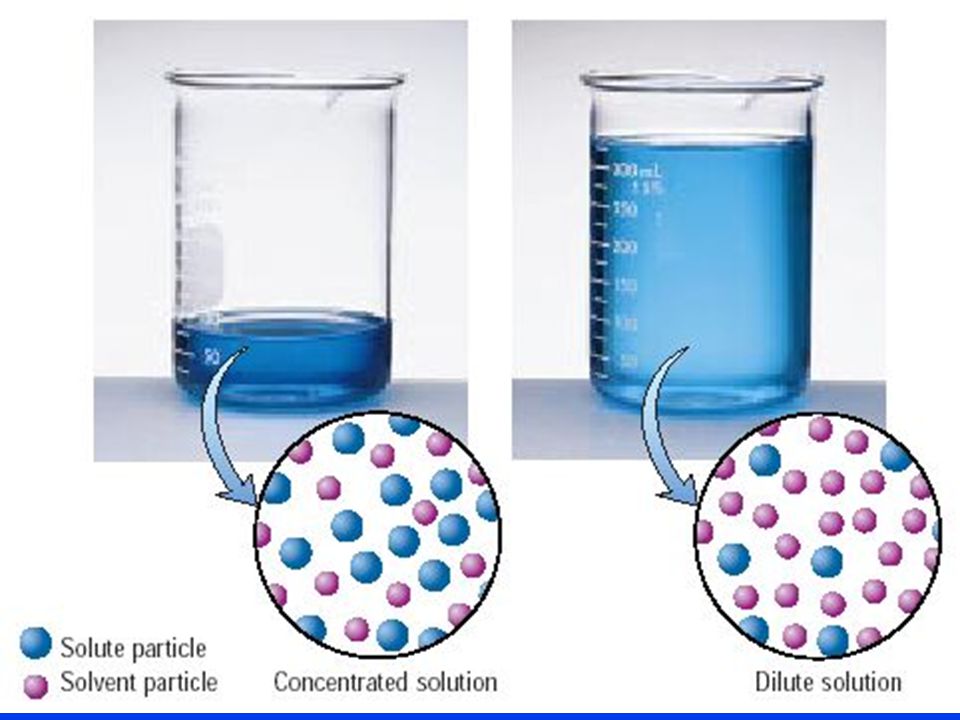
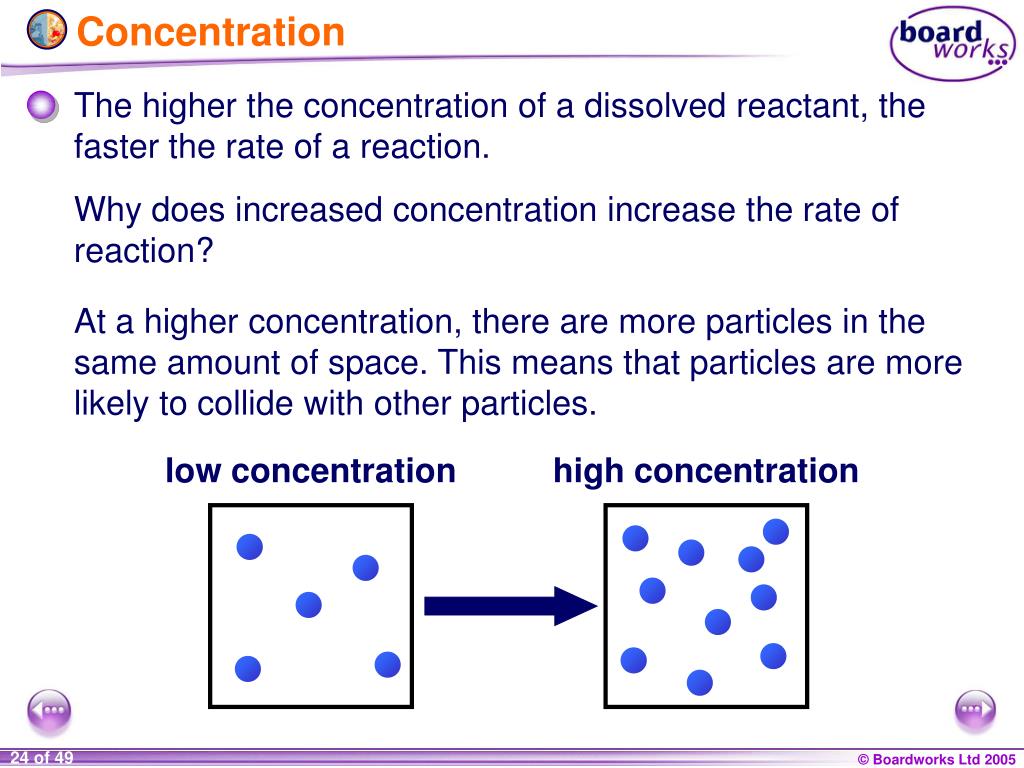
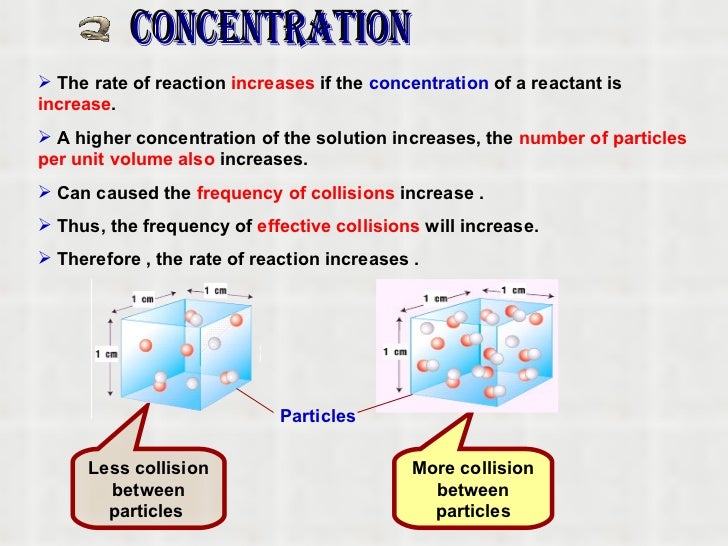
How to increase concentration of a solution. The dissolution of soluble substances such as mgcl 2 and caso 4 within the pore structure led to an increase in the concentration of ca 2+ and mg 2+ ions,. When increasing 90 % × 100 = 80(%) × a concentration use the initial and desired % amounts of the base, not the. 22.4 ghcl × 1 molhcl 36.5 ghcl = 0.614mol hcl 22.4 g h c l × 1 m o l h c l 36.5 g h c l.
The concentration of a solution can be calculated using: The role of humans. Human activity is the cause of increased greenhouse gas concentrations.
Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Over the last century, burning of fossil fuels like coal and oil has. Method 1 using the mass per volume equation download article 1 find the mass of the solute mixed in with the solvent.
The mass of dissolved solute in grams, g the volume of solution (or solvent) in cubic decimetres, dm 3 key fact \. Often, a worker will need to change the concentration of a solution by changing the amount of. You get the mass of solute for the solution, mix the solute with a known volume of solvent, and divide mass by volume for concentration.
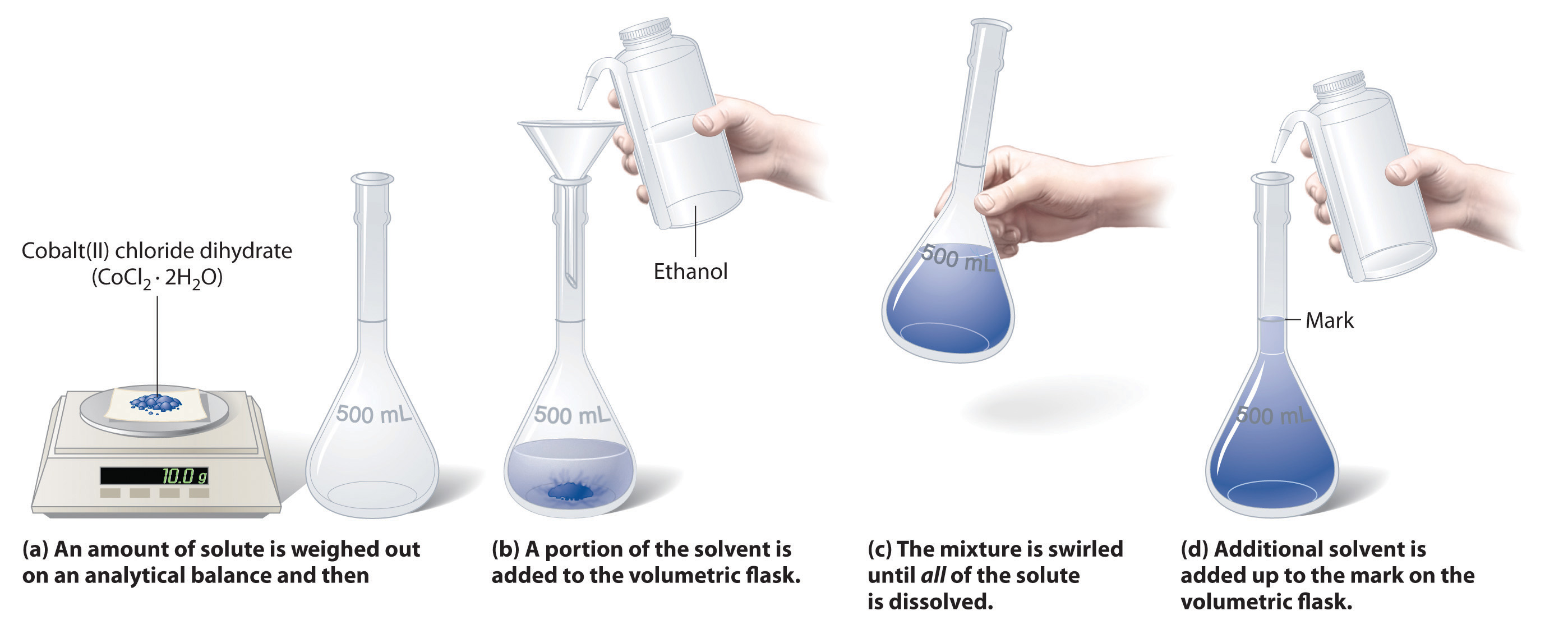
The concentration calculator is a tool for converting the molarity into percentage concentration (or vice versa) with a known molar mass of the dissolved. Use c1 x v1= c2 x v2 percentages cancel out nb: Solutions of known concentration can be prepared either by dissolving a known mass of solute in a solvent and diluting to a desired final volume or by diluting the.
That will be the volume by volume percentage of a solution. You will usually see chemists working with the number of moles. It can be used when we are mixing two liquids to form a solution
What are all the ways you can change the concentration. First, convert the mass of solute to moles using the molar mass of hcl (36.5 g/mol): The solute is the substance that you’re.
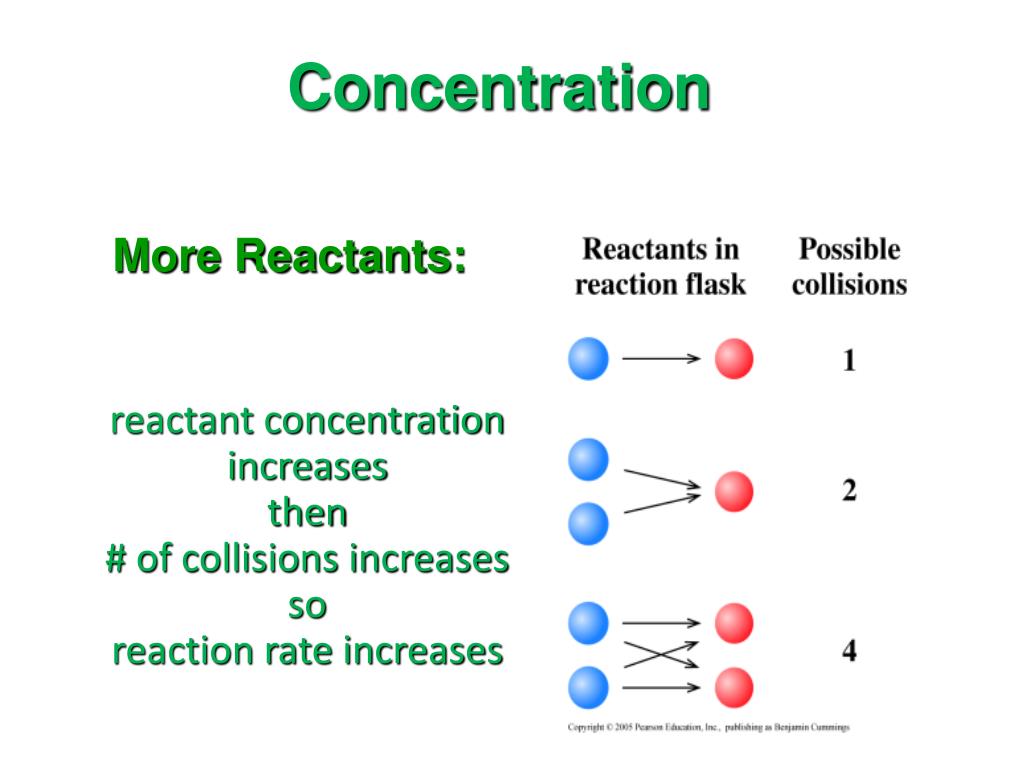
Learn how to dilute and concentrate solutions. If the concentration of a. Le chȃtelier’s principle can be used to predict the effect that a stress like changing concentration has on a reaction system at equilibrium.
Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Sometimes, by modifying the quantity of solvent, a worker would need to modify the concentration of a solution. Then check molarity with the concentration meter.
Dilution is the addition of a solvent that reduces the solute. In this case, the concentration of \(\ce{hi}\) gradually decreas… Watch your solution change color as you mix chemicals with water.


.PNG)